Digital Transformation in Pharma Manufacturing With UNS, MQTT & Distributed Data Intelligence
Transform pharma manufacturing with UNS, MQTT & Distributed Data Intelligence. Break silos, boost compliance, and drive Pharma 4.0 innovation.
Pharmaceutical manufacturing faces several pressures such as supply chain disruptions, quality control issues, and increased regulatory control that are impacting the ability to bring safe and effective medicines from R&D to market faster. Pharma Manufacturing is increasingly turning to the integration of digital technologies and automation, or Pharma 4.0, to solve these problems.
Automate processes and use data analytics to improve efficiency across manufacturing lines and locations.
Use digital technologies to be more agile and quickly identify and recover from costly mistakes or unsuccessful endeavors.
Apply real-time monitoring and analytics to quickly identify and fix issues before they negatively affect quality.
Automate regulatory reporting and quality control, and ensure the traceability of data to support regulatory compliance.
The pharmaceutical manufacturing industry faces several pressures that are impacting its ability to bring safe and effective medicines from R&D to market faster and overcome issues like supply chain disruptions and competition. The pharmaceutical world is highly regulated and compliance management is both time-consuming and costly, while non-compliance can lead to significant fines or legal actions from organizations like FDA and the European Medical Agency. The Food and Drug Administration, for example, has the FDA 21 CFR Part 11 which dictates that electronic records of all drug manufacturing data needs to be available in raw uninterpreted format for at least 7 years for audits.
Pharma manufacturers are increasingly turning to the integration of digital technologies and automation to solve these challenges. This approach has been termed Pharma 4.0, which involves the use of advanced technologies such as artificial intelligence, machine learning, robotics, and Industrial IoT to improve operations and ensure compliance.
HiveMQ has been the cornerstone of our transformation across 15 pharmaceutical factories worldwide. With zero data loss and impeccable reliability, we've met stringent FDA requirements effortlessly.
Confidential
/Pharma Company
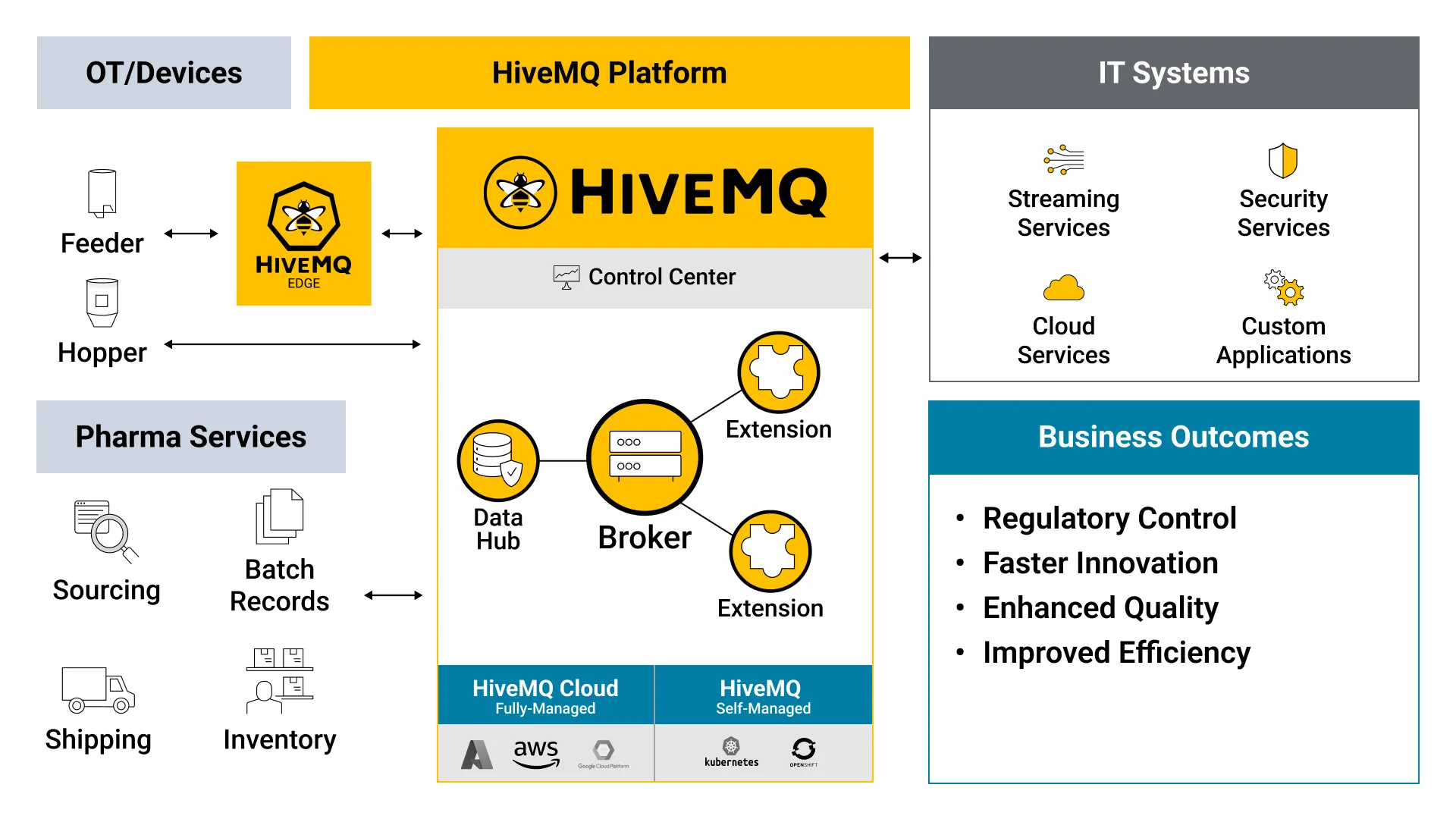
HiveMQ solves the challenges of data collection and movement in Pharma manufacturing by adding a reliable, scalable and secure data abstraction layer between OT and IT systems that enables heterogeneous machines and processes to work together seamlessly even in constrained environments.
Operate mission-critical systems reliably 24/7 with zero message loss and redundant clustering technology.
Ensure applications and data meet the highest security standards with end-to-end encryption and configurable security controls.
Add any number of sites and scale to millions of connected devices seamlessly with a linear design for scalability
Troubleshoot and keep all factory systems running as planned with tools and metrics for transparency and observability
Focus on your core business instead of using developer resources with OT-IT data integration into enterprise applications and infrastructure like Apache Kafka.
Achieve rapid time-to-value with a platform that is flexible enough to deploy on-premise, in any cloud, or via the fully-managed and feature-rich HiveMQ Cloud offering.
HiveMQ prices its offerings based on the value you derive, not on speed and feeds. There is no need to count messages or integrations, rather we align to specific business goals and outcomes. Specifically, we offer pricing based on:
Request Quote
How many plants or locations do you need to support and over what time period?
How many devices and protocol types need to be supported per plant?

Do you need us to run the software for you, or will you self-manage the deployment? What is the relationship between plants and headquarters?

Are you looking for a multi-year deal or to renew each year?
Transform pharma manufacturing with UNS, MQTT & Distributed Data Intelligence. Break silos, boost compliance, and drive Pharma 4.0 innovation.
Learn how Lilly leverages HiveMQ to streamline data integration for regulatory compliance and digital transformation.
Download this Pharma Manufacturing data sheet to learn why HiveMQ is the most trusted enterprise MQTT platform for Pharma 4.0.
Optimize supply chain, regulatory compliance, & modular automation for your Pharma Manufacturing with MQTT Sparkplug-powered IIoT technology.
Discover how the leading pharmaceutical company achieved excellence in pharma manufacturing and compliance through HiveMQ's advanced IoT solutions. Dive into our case study to explore the seamless integration of smart manufacturing, elevating connectivity, performance, and compliance standards in the pharmaceutical industry.
Discover the journey of IIoT data in Pharma manufacturing from collection to digital maturity & how it empowers digital transformation along the way.
Choose between a fully-managed cloud or self-managed platform. Our experts can help you with your solution and demonstrate HiveMQ in action.