Empowering Digital Transformation in Pharma Manufacturing through Pharma 4.0
Introduction
The global Pharmaceutical Manufacturing market is expected to grow at a CAGR of almost 13% to $1,190 billion by 2030 according to Precedence Research. However, despite strong projected revenue performance, industry profits during the same period are expected to decrease due to mounting competition from lower-cost generic drugs, biosimilars, and expiring patents of several blockbuster drugs according to IBISWorld.
Pharmaceutical manufacturing faces several additional pressures that are impacting their ability to bring safe and effective medicines from R&D to market faster, from supply chain disruptions to regulatory requirements. Compliance management alone is time-consuming and costly, and non-compliance can lead to significant fines or legal action. The industry needs to solve these challenges in a cost-effective manner without compromising on quality and efficiency.
Introducing Pharma 4.0
The pharmaceutical world is increasingly turning to the integration of digital technologies and automation to solve these problems. The approach has been coined as Pharma 4.0, which is a concept that is borrowed from Industry 4.0. Pharma 4.0 involves the use of advanced technologies such as artificial intelligence, machine learning, robotics, and Industrial IoT (IIoT) to improve efficiency, quality, and safety in drug R&D, manufacturing, and distribution. The goal of this initiative is to:
Improve efficiency: Automate the entire process across manufacturing lines and locations.
Enhance quality control: Apply real-time monitoring and analytics to quickly identify and fix issues before they become endemic in the process.
Innovate faster: Use digital technologies to respond to change faster, and quickly identify and recover from costly mistakes or unsuccessful endeavors.
The hope is that this type of digital transformation will allow high-quality, cost-effective medicine to be brought to the market faster so that pharma companies can focus more on the research of new drugs.
Although Pharma Manufacturing has begun to warm up to IIoT and its potential, GEP says that only 30% of the top 20 pharma companies have adopted some level of IIoT technologies in their manufacturing process. This leaves a lot of potential value to be gained in Pharma Manufacturing by properly deploying IIoT solutions to improve operations.
Data and Pharma 4.0
Collecting, understanding, and analyzing data is key to adopting Pharma 4.0. Pharma companies generate vast amounts of data daily, from lab experiments to manufacturing to supply chain management. This data has the potential to help Pharma manufacturers make better-informed decisions, optimize processes, and drive growth in the following areas.
Compliance
Pharmaceutical is probably the most highly regulated industry, and compliance with regulations such as Food and Drug Administration (FDA), Medicines & Healthcare products Regulatory Agency (MHRA), European Medicines Agency (EMA), and others can be extremely challenging. Ensuring compliance requires accurate data tracking, zero data loss, secure storage, and reporting, all of which is challenging without data automation. For example in the US, FDA has the 21 CFR Part 11 that states that electronic records of all drug manufacturing data needs to be available in the raw uninterpreted format for at least 7 years for audits.
An effective compliance program is essential to prevent business disruptions and loss of reputation from failed audits. As a result, Pharma manufacturers must maintain strict quality control, detailed product information, digital batch records, and continuous data integration between operational technology (OT) and information technology (IT) systems.
Operational Efficiency
Operational efficiency refers to the enablement of high visibility, informational transparency, robust security, effective work management and process tracking in Pharma manufacturing. As there are a number of factors that need to be taken into consideration, it has become more and more challenging for Pharma manufacturing companies to achieve operational efficiency.
One way to make improvements is by applying sophisticated algorithms on the process data, which has been possible with better computational power and storage capabilities. Instead of intuition, the new normal is to rely on data to drive digital innovations and operational efficiency decisions. Indeed, data is the most valuable resource for Pharma manufacturing organizations.
Quality Control
The aim of quality control in Pharma manufacturing is to verify and test the medicine at various stages of production, to ensure every product is of the highest quality. Quality control also involves identifying any defects in products and fixing these problems with corrective techniques and measures. Data enables the tracking of quality measurements, ensures conditions are optimal for production, and more.
Pharma 4.0 – The Journey
As shown in Figure 1, the journey from data collection to digital maturity in Pharma manufacturing is one in which analysis, context, and insights are added to transform raw data captured from a device or system into information, knowledge, and, finally, actionable wisdom for decision makers.
Figure 1: The stages of the data maturity model on the path to realizing Pharma 4.0.
First, data is collected from Pharma manufacturing machines/processes like feeding machines, wet granulation machines, flatbed dryers, compressors, lyophilizers, and similar equipment. They are then normalized, digitized, and organized as Big Data. Next, meaning (or labeling) is added, and data is synthesized into knowledge via AI. Finally, the data is transformed into actionable wisdom attained through the combined insights of digital maturity.
Data Collection - The First Frontier
The first and most significant frontier to achieve digital maturity for Pharma 4.0 is data collection and movement. Data from Pharma manufacturing machines, processes, and applications are captured and stored via key ingestion technologies. On the operational technology (OT) side, data is stored with controllers, PLCs, gateways, and edge devices, and on the IT side, it is stored in a data center or enterprise cloud. Data storage technology enables the long-term storage of digitized data captured from advanced sensors and systems. This data-rich environment enables advanced initiatives such as machine learning, AI, adaptive control, and digital twins.
Pharma Data Collection Challenges
There are some challenges to data collection and data movement in Pharma manufacturing. Machines and processes in the Pharma manufacturing plant are heterogeneous and use various protocols to communicate. Data generated during Pharma manufacturing can vary significantly in format, quality, and completeness. This can make collecting, integrating, and analyzing data difficult.
Data connectivity is also a major issue due to factory systems’ archaic, legacy nature. As a result, IT and OT systems typically don’t have an easy way to communicate to enable Pharma 4.0 initiatives.
Industrial IoT (IIoT), a subset of Pharma 4.0, uses smart sensors and actuators along with software to consolidate the data to enhance manufacturing and industrial processes. IIoT is a key enabler in achieving OT IT convergence and the interoperability of the various communication protocols. It creates a data abstraction layer in the middle and a common data language to translate the various communication protocols to enable interoperability.
 Figure 2: IIoT serves as the intersection point between OT and IT systems
Figure 2: IIoT serves as the intersection point between OT and IT systems
OT IT convergence matters because success in today’s connected industrial landscape hinges on collaboration. IIoT is changing how manufacturers work, blurring the lines between IT and operations. For example, IT pros might now spend more time working with equipment on the factory floor, while OT teams must focus on cybersecurity and networking best practices. IT-OT convergence isn’t about turning IT pros into heavy machinery operators or plant engineers into data scientists. Instead, it’s about creating a strategy that bridges the gap and allows organizations to improve operational performance by working around a unified set of objectives and KPIs.
Pharma Data Collection Solution - MQTT and a Data Broker
A Data Broker is a key technology enabler for IIoT to enable this data abstraction layer. A data broker is an intermediary entity that enables OT and IT client systems to communicate with each other. Using an underlying standard such as MQTT, a data broker supports the ability to connect multiple clients publishing data and multiple clients that are subscribed to receive the data such as enterprise applications. The clients communicating with the broker can abstract the underlying protocol that the machines/processes use to communicate. The broker works well in low bandwidth environments with unreliable communication mechanisms due to the underlying publish/subscribe method, where machines/processes don’t need to keep polling to get the data.
MQTT is a standard binary publish-subscribe messaging protocol designed for fast and reliable data transport between devices, especially under very constrained conditions. Constraints include unreliable network connectivity, limited bandwidth, limited battery power, and similar limited conditions. It is built on top of TCP/IP, which is the go-to communication protocol to interconnect network devices on the Internet. MQTT is ideal for IIoT due to the reasons mentioned above.
Figure 3: How an MQTT messaging system works
The MQTT data broker can securely communicate the data between publishing clients, typically on the OT side, to subscribing clients on the IT side. For example, an MES application might want data from the SCADA system to run its analytics to identify batch variability easily. The MES application would run an MQTT client subscribed to the broker. The SCADA client would publish data to the broker when available. As a result, the MES application subscribed to the broker would automatically get the updates without needing to poll for the data.
MQTT technology is designed to push data to and from thousands of remote devices across numerous sites to the enterprise. Sparkplug is a framework that sits on top of MQTT to add more context to the Manufacturing data. It is an open-source software specification that provides MQTT clients with a framework to integrate data and provide context by defining data models. It provides a consistent way for Pharma manufacturing equipment manufacturers and software providers to share contextual data, accelerating the digital transformation of existing operations.
Sparkplug allows IIoT deployments to decouple the data between hardware and software sources. With Sparkplug, new data sources are immediately discoverable to other system components, and these sources can become a single source of truth. Sparkplug is fully secured, requiring no open ports for new devices and requiring TLS for all data transport.
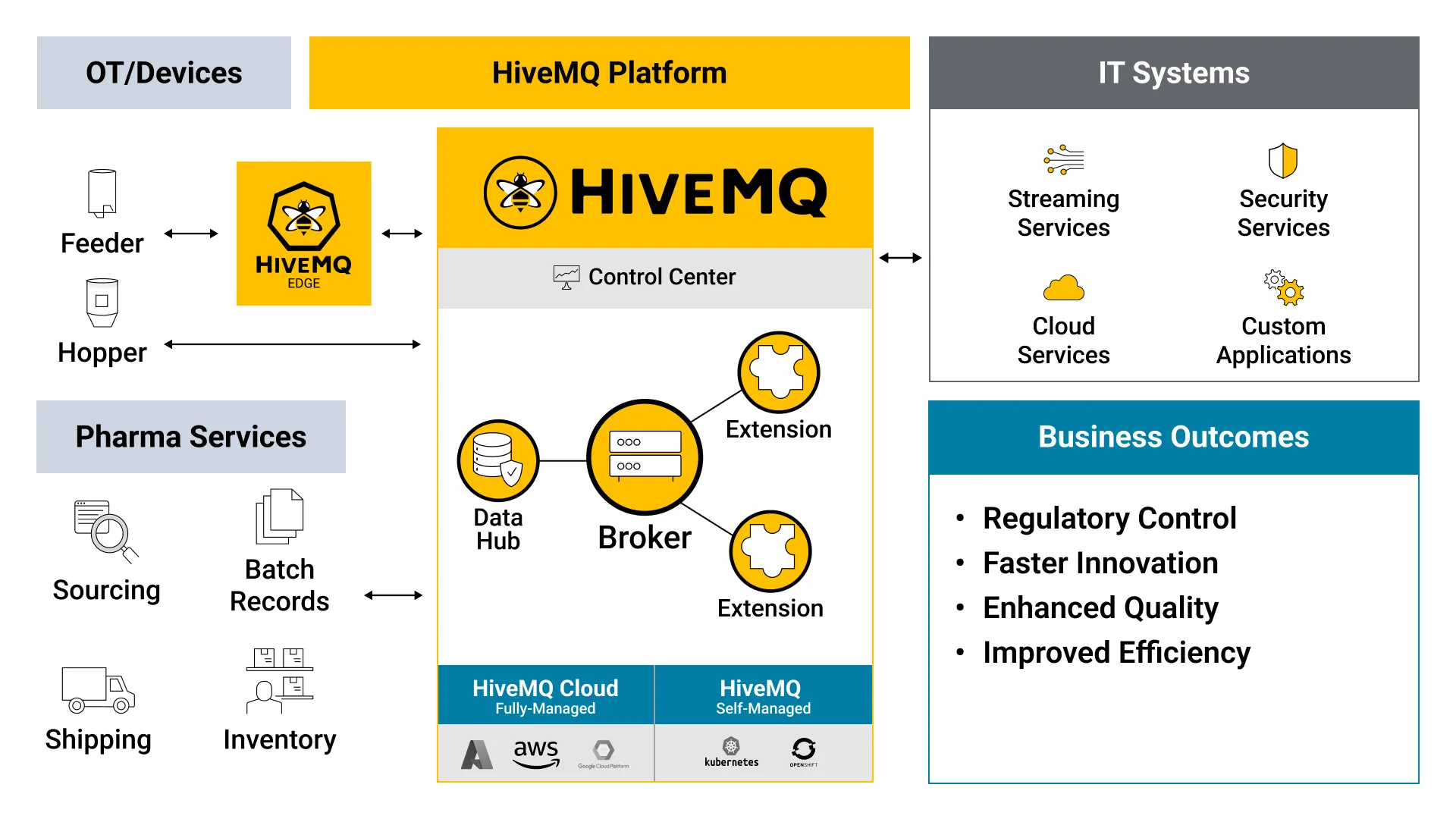
HiveMQ: An Enterprise MQTT Platform for Enabling Digital Transformation for Pharma Manufacturing
HiveMQ is an MQTT-based enterprise messaging platform designed for fast, efficient, and reliable movement of data to and from manufacturing machines, processes, applications, and supply chain components to enterprise data locations on premises or in the cloud. Adopting the HiveMQ broker enables customers to optimize supply chains, automate regulatory compliance reporting, improve operational efficiency and enhance product quality which includes enabling digital batch records.
Pharma Manufacturing IIoT Use Case Reference Architectures
Here are common architectures for supply chain optimization and regulatory reporting use cases seen in Pharma Manufacturing:
Use Case 1: Pharma Supply Chain Optimization
Pharma supply chain managers want to trigger necessary workflows as needed from a centralized location based on leading indicators derived from sourcing, supplier inventory and shipping. Supply chain performance data flows through the HiveMQ Broker to the cloud provider’s infrastructure where it is ingested and then analyzed or monitored.
 Use Case 1: Pharma Supply Chain Optimization
Use Case 1: Pharma Supply Chain Optimization
There are two major advantages to using a HiveMQ Broker in this scenario. The first is that it provides a very scalable solution that load balances the control data from different systems that may be in remote locations, worldwide. Secondly, it offers tools to provide a high level of factory IIoT data observability and transparency to overcome any data bottlenecks going to the cloud or coming back to remote locations.
Use Case 2: Regulatory Reporting Enablement
Regulatory scrutiny and enforcement actions will certainly continue given the ever increasing volume of drug sales by Pharma companies. The industry will be challenged to move beyond the current crisis management approach to regulatory compliance and implement a comprehensive strategic approach that builds compliance into the way companies do business.
The batch records, which maintain all the details of the manufacturing steps and are typically manual, will need to be digitized. HiveMQ can help ensure that the Manufacturers are FDA 21 CFR Part 11 and/or European Medical Agency audit compliant by making sure that the manufacturing batch records data, along with other factory data, flows through the HiveMQ Broker to the cloud provider’s infrastructure. Here the raw data is stored in data lakes from where automated reports and dashboards can be created.
 Use Case 2: Regulatory Reporting Enablement
Use Case 2: Regulatory Reporting Enablement
In this use case, the HiveMQ Broker is highly scalable and balances the load of the control data and process data being received from various locations. In addition, it provides highly secure communication between HiveMQ and the remote systems or cloud with features like TLS/SSL support, OAuth 2.0, X.509 certificates, etc.
Next Steps: Explore HiveMQ’s Solution for Pharma 4.0
As discussed, the move to Pharma 4.0 is real and a business-critical initiative. Data is the most valuable in Pharma manufacturing, forming the basis and yardstick for improving efficiency, quality, and safety in drug development, manufacturing, and distribution. At HiveMQ we have helped many Pharma companies embark on their journey to Pharma 4.0 and welcome the opportunity to discuss a solution tailored to your business needs.
